Stainless Steel
Stainless steel, as its name suggests, is well known as steel that resists staining. By adding at least 12% chromium to iron, a natural oxide film forms on the surface, protecting it from rust.
Its history is relatively recent, beginning in the 18th century with the discovery of chromium in ore. Research on alloys progressed in Europe, leading to chromium-iron alloys in the 19th century, and by the 20th century, “rust-resistant iron” was developed by mixing chromium and nickel into iron.
In Japan, stainless steel became widely recognized during the rapid economic growth of the 1950s and 60s, when it was used in kitchen sinks for new suburban housing developments. Since then, it has found its way into bathtubs, architectural materials, and various everyday applications.
Stainless steel is characterized by its distinctive silver color. While coloring methods like painting and plating have been tried, these often suffered from peeling and fading, limiting durability.At Abel, we focused on the oxide film naturally forming on stainless steel surfaces. By electrically and chemically growing this film’s thickness and using light interference, we achieve a rich black finish that is both durable and integral to the metal itself.
References:
“SUS ga Kuroobi Kinzoku Urushi — Eigiki to Nazukerareta Otoko: Sono Myōmyōtaru Ikizama” by Hideki Iai“The History of Stainless Steel Invention” by Takashi Suzuki (Agne Technical Center)

Oxide Film Composition
Properties of Stainless Steel

Austenitic Stainless Steel
18%Cr-8%Ni
(SUS304)

Ferritic Stainless Steel
18%Cr
(SUS430)

Martensitic Stainless Steel
12%Cr
(SUS410)
| Type | Characteristics |
|---|---|
| 304 Series |
• High-strength stainless steel |
| 316 Series |
• Pitting corrosion resistance |
| 430 Series |
• Heat and oxidation resistance |
Stainless Steel and Abel Black®
Stainless steel is characterized by its silver color. Attempts to color it through painting or plating often faced issues such as peeling and fading, limiting color durability. Abel Co., Ltd. focused on the oxide film naturally covering the surface of stainless steel. By growing this oxide film through the combined effects of electricity and chemicals, and utilizing light interference, it became possible to perceive a deep black color.
Properties of Stainless Steel
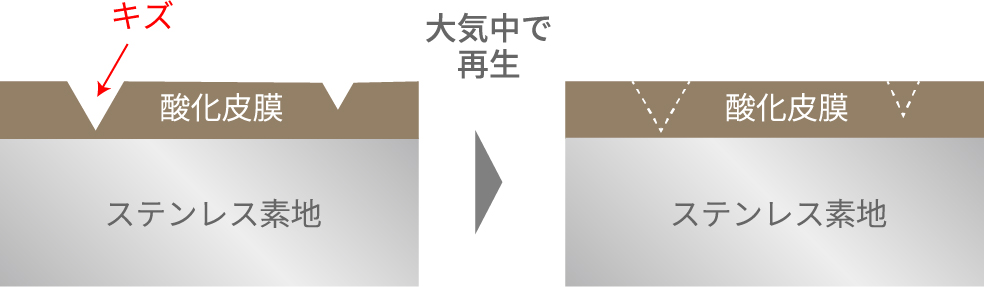
To improve corrosion resistance and mechanical properties, elements such as nickel and molybdenum are added to stainless steel. A protective passive film forms on its surface. This passive film, composed mainly of iron and chromium oxides (or hydroxides), is extremely thin—less than one hundredth of one micron. Though easily damaged mechanically, exposing the bare metal, it spontaneously repairs itself upon contact with oxygen, water vapor, or moisture, maintaining its corrosion resistance.
Magnetic Properties of Stainless Steel
Some stainless steels are magnetic, while others are not. 18-8 (SUS304) is non-magnetic, whereas 18Cr (SUS430) is magnetic. However, SUS304 can become magnetic after processing. This difference stems from their crystal structures: austenitic stainless steels are non-magnetic, while ferritic and martensitic types are magnetic. When SUS304 is worked, part of its austenitic structure transforms into martensitic, causing it to become magnetic.